Claim: Pakistan is using a Chinese vaccine which was proven to have an efficacy of 50.4% during late-stage trials held in Brazil.
Fact: Pakistan has currently approved Sinopharm Biotech Ltd.’s vaccine from China. The Sinovac vaccine that was tested in Brazil has not been approved in Pakistan. Pakistan is currently testing one other Chinese vaccine, which is CanSino’s vaccine, also separate from Sanivoc’s.
On 21 January, Minister of Foreign Affairs Shah Mahmood Qureshi announced that his Chinese counterpart, Wang Yi, had promised Pakistan 500,000 complimentary doses of the BBIBP-CorV vaccine developed by the state-owned Sinopharm company.
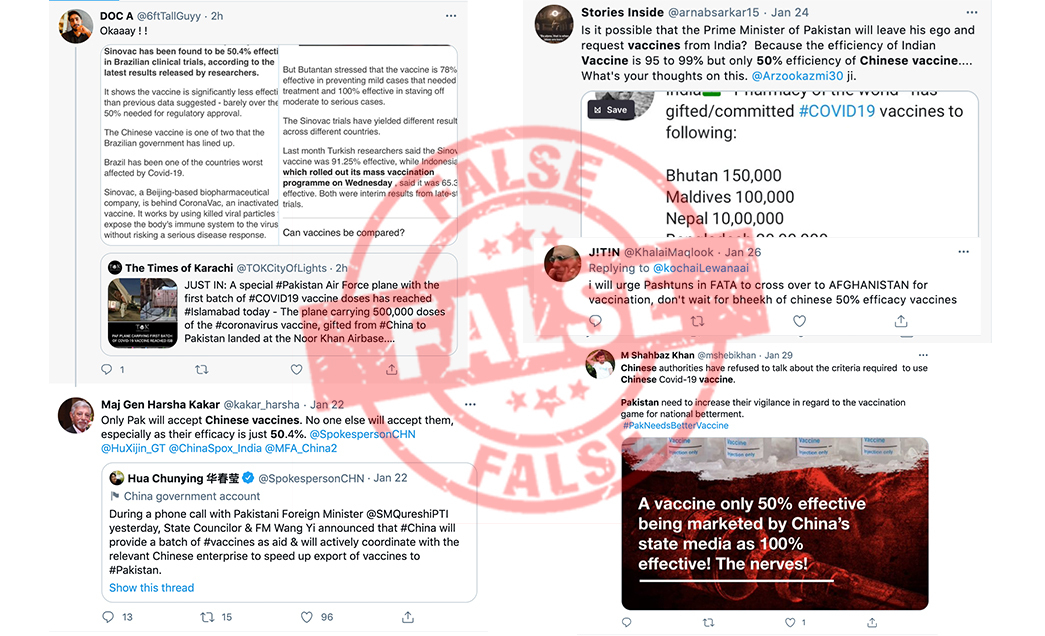
Since then social media has criticized the vaccine, citing results released earlier this month in Brazil, according to which the vaccine developed by China-based Sinovac Biotech is 50.4% effective. A Pakistani news media outlet also stated that Shah Mahmood Qureshi had referred to Sinovac’s vaccine in his statement.
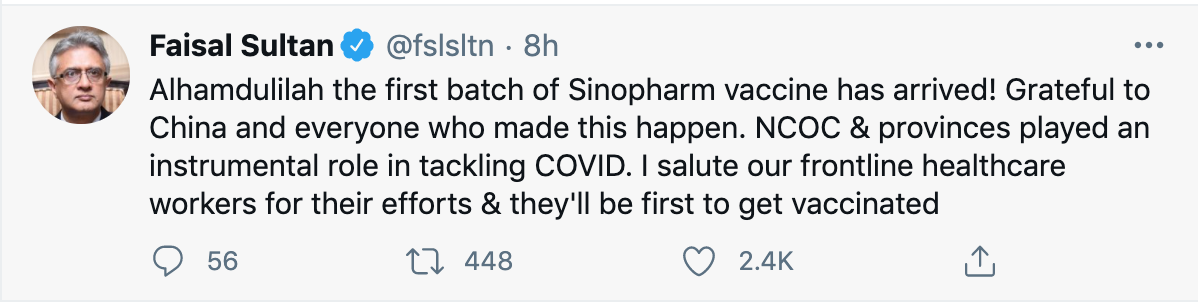
Soch Fact Check found that Pakistan received 0.5 million doses of Sinopharm Biotech Ltd’s vaccine from China on 31 January. Pakistan did not receive Sinovac’s vaccine, which has been undergoing separate testing, and was shown to be 50.4% effective as per interim results released by Brazil.
Chinese Vaccines in Pakistan
Sinopharm Biotech Ltd
In September 2020, Pakistan signed up for trials of vaccines being produced by China National Biotec Group Co. (CNBG), a subsidiary of China’s state-owned Sinopharm Biotech Ltd. Dr Ghazna Khalid, clinical lead for the trial who is also on the government-appointment Scientific Taskforce for Covid-19, told Soch Fact Check that the trial is being headed by the International Centre for Chemical and Biological Sciences. Dr Khalid confirmed that the trial is currently ongoing, with the second dose of the vaccine due to be administered sometime next week. The trial is based on a sample size of hundreds, as is the procedure with Phase 1 trials, Dr Khalid stated.
On 18 January, the Drug Regulatory Authority Pakistan (DRAP) granted Sinopharm’s vaccine for emergency use approval. Speaking to Soch Fact Check Dr Faisal Sultan, special assistant to the prime minister on national health services, stated that DRAP granted approval after evaluating results from late-stage trials of the vaccine carried out in the UAE.
Chaudhry Fawad Hussain, Pakistan’s minister for science and technology, had already announced in December that Pakistan will purchase 1.2 million doses of Sinopharm’s vaccine. As mentioned above, on 21 January, Shah Mehmood Qureshi announced that his Chinese counterpart had promised Pakistan 0.5 million complimentary doses of the drug. This batch was transported by plane from Beijing to Islamabad on 1 February. On 2 February, Pakistan began its vaccination drive.
Update: On 4 January, while speaking to media, Dr Sultan announced that Sinopharm’s vaccine is not recommended for people below the age of 18 and above 60 years old.
How Effective is the Sinopharm Vaccine?
Pakistan is one of ten countries where Sinopharm tested the two vaccines that it has been developing. Pakistan signed up to test the BBIBP-CorV, which was also being tested in the UAE. These include Bahrain, Morocco, Argentina and Jordan.
On 9 December, the United Arab Emirates’ health ministry announced its approval for one of Sinopharm’s vaccines, the same one that was later approved in Pakistan, citing interim results from its late-stage trials of the medicine. The vaccine had shown 86% against coronavirus infection, according to a statement released by the health ministry, and 100% effectiveness in “preventing moderate and severe cases” of Covid-19. These results were based on a sample set of 31,000 participants across 125 nationalities, between the ages of 18 and 60, who took two doses over 28 days.
On 12 December, the Peruvian government announced that they were suspending trials for Sinopharm due to a “serious adverse event” with one of its volunteers. The suspension was, however, lifted a few days later. At this point, there is no further information available in the public domain regarding the suspension of the trial, however, the Peruvian government authorized the vaccine for emergency use on 26 January and is set to start its vaccination drive on 10 February.
On 30 December, CNBG, the company that developed the Sinophram vaccines, announced that interim analysis from Stage 3 trials had shown a 79.3% efficacy for the same vaccine as that was approved in the UAE. This result was based on a sample set of 60,000 participants. Speaking to Global Times*, a tabloid published by China’s ruling Communist Party, CNBG said the varying results are due to differing methodologies. Other news outlets that reached out to the company were met with no comment.
On 31 December, China approved Sinopharm’s vaccine. Egypt and Hungary have also followed suit, amongst others. Sinopharm, much like Sinovac, has been criticised for not providing more detailed insight into methodology and results’ breakdown.
CanSino Biologics Inc
Phase 3 trials for a vaccine being developed by China’s CanSino Biologics Inc launched in Pakistan in September. The trial was conducted by the government-run National Institute of Health, in collaboration with CanSino’s local partner, AJM Pharma Pvt. The initial sample size goal of 10,000 volunteers was later increased to 17,500 participants.
DRAP, who allowed the trial to be conducted, have yet to announce approval for this vaccine. In January, Hassan Abbas Zaheer of AJM Pharma, told the media that CanSino would be providing Pakistan 20 million doses of its vaccine on a preferential basis. Dr Faisal Sultan, special health assistant to the prime minister, also confirmed this while speaking to the media.
CanSino’s late-stage trials included over 40,000 participants in five countries. In a statement released to Global Times*, a magazine published by China’s ruling party, on 1 February, the company stated that they are expecting interim results from these countries in the coming week. The vaccine has met its pre-specified efficacy and safety criteria as per current analysis and no serious adverse events have occurred during the course of the trials.
Sinovac Biotech Ltd: Piecemeal results inapplicable to Pakistan
Pakistan has not approved the use of the Sinovac’s vaccine, however, many Pakistanis online are criticising the government for procuring vaccines from China, citing Brazil’s trial results as proof of the drug being inferior to Western counterparts.
Brazil was the first country to complete late-stage trials of the vaccine developed by China-based Sinovac Biotech. Its state-run Butantan Institute was supposed to release results in early December, but postponed doing so several times. On 23 December 2020, officials from the institute stated that Sinovac had requested they do so till the company had combined results from global trials. Officials did confirm that the trial, conducted on 13,000 volunteers, had marked Sinovac’s vaccine as being viable for emergency use approval by Brazil’s health regulator, Anvisa. As per Anvisa, a vaccine must display minimum 50% efficacy for approval. Anvisa did not, however, approve the vaccine at the time.
The very next day, on 25 December, Turkey announced that Sinovac’s vaccine was 91.25% effective, based on interim results of their late-stage trials. Their sample size, however, was smaller, at approximately 7,000 volunteers, and their interim conclusion was based on an even smaller data set of 1,322 people. Expounding on results, researchers explained that of the 29 people who had been infected, 26 had been given placebos. Results had been evaluated two weeks after the second doses had been administered. Turkish authorities had initially planned on releasing results after 40 people from the sample were infected with the Coronavirus, but had chosen to release an interim analysis as the country was considering granting the vaccine emergency use approval.
Early in December, Sinovac’s Indonesia partner, Bio Farma, initially announced that interim data from trials showed 97% efficacy. Sinovac later clarified that this figure referred to the seroconversion rate, which does not necessarily correspond with efficacy, thus no conclusions could be drawn yet about the vaccine’s efficacy from Indonesia’s trials. Bio Farma later confirmed this.
On 11 January, Indonesia became the first country outside of China to grant Sinovac’s vaccine emergency use approval. It’s drug regulatory authority, BPOM, announced that late-stage trials had shown interim results of 65.3% efficacy. BPOM’s head said that, from its sample size of 1,600 volunteers, this result was based on 25 who had been infected with COVID-19. No other details were released.
Brazil eventually released its results on 7 January, announcing that late-stage trials had shown an efficacy of 78% in “mild to severe cases”, with the vaccine offering total immunity from severe cases of the Coronavirus. In a sample of about 13,000 volunteers, 218 had become infected with COVID-19, with just over 160 of these having received a placebo. No other details were revealed.
On 11 January, Brazil’s news media quoted anonymous sources as stating that the trials had, in fact, shown an efficacy of less than 60%. While medical experts in the country were already criticising the previous week’s announcement as not being transparent in its disclosure of details, the Butantan Institute dismissed the news media’s report as being “speculative”.
On 12 January, however, Butantan Institute’s researchers announced that previous results did not take into account data from a set of “very mild” infections. Factoring in this date has reduced the vaccine’s efficacy to 50.4%. They stressed that the 78% efficacy remains in preventing mild cases of COVID-19, and the vaccine 100% effective in preventing moderate to severe cases. Anvisa gave Sinovac emergency use approval on 16 January.
The piecemeal release of Sinovac’s late-stage trials, as well as a lack of transparency regarding trial details, has attracted criticism from medical experts, who are calling for greater scrutiny towards Chinese vaccines. Despite this, over 380 million doses of the Sinovac vaccine have been ordered.
Summary: On 11 January, Brazil’s Butantan Institute announced that a Chinese vaccine it had been testing had shown an efficacy of 50.4%. This led many in Pakistan to believe that the country has approved the same vaccine as that which was tested in Brazil. Pakistan has currently approved Sinopharm Biotech Ltd.’s vaccine from China. The Sinovac vaccine that was tested in Brazil has not been approved in Pakistan. Pakistan is currently testing one other Chinese vaccine, which is CanSino’s vaccine, also separate from Sinovoc’s.
*Global Times, run by the China’s ruling Communist Party, has been criticised for spreading disinformation regarding coronavirus vaccines and, in some cases, being biased towards the state’s official narrative regarding the Covid-19 outbreak.

This is really good investigative journalism. Thanks alot, I feel much relieved